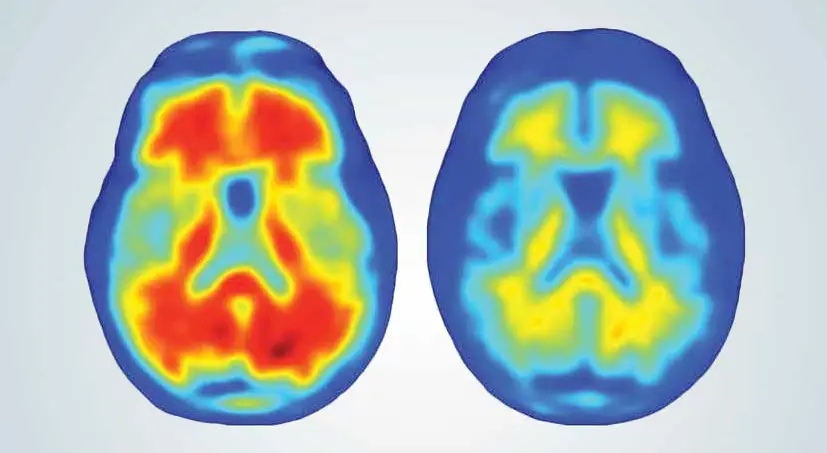
4th September 2016 A promising new treatment for Alzheimer's A new antibody has been shown to substantially reduce the harmful beta-amyloid plaques in patients with early-stage Alzheimer's. Its makers have described it as "the best news for dementia in 25 years."
Aducanumab, a new antibody developed by the University of Zurich (UZH), has been shown to trigger a meaningful reduction of harmful beta-amyloid plaques in patients with early-stage Alzheimer's disease. These protein deposits in the brain are a classic sign of Alzheimer's disease and contribute to the progressive degeneration of brain cells. Furthermore, the researchers demonstrated in an early stage clinical study that – after one year of treatment with Aducanumab – cognitive decline could be significantly slowed in antibody-treated patients, as opposed to the placebo group. Although the precise causes of Alzheimer's remain unknown, it is clear that the disease commences with progressive amyloid deposition in the brain approximately 10 to 15 years before the clinical symptoms such as memory loss. Researchers have now been able to show that Aducanumab, a human monoclonal antibody, selectively binds amyloid plaques, thus enabling microglial cells to remove them. A one-year trial, as part of a phase Ib study, resulted in almost complete clearance of the harmful plaques in treated patients. The results, which also involved biotech company Biogen and the UZH spin-off Neurimmune, were published this week in the journal Nature. "This is the best news that we have had in our 25 years," said Dr Alfred Sandrock from Biogen, which is based in Massachusetts. "It brings new hope to patients with this disease." "The results of this clinical study make us optimistic that we can potentially make a great step forward in treating Alzheimer's disease," said Prof. Roger M. Nitsch from the Institute for Regenerative Medicine at the UZH. "The effect of the antibody is very impressive. And the outcome is dependent on the dosage and length of treatment." As shown in the image above, practically no beta-amyloid plaques could be detected in patients who received the highest dose of the antibody. The drug was developed using a technology platform from Neurimmune. Using blood collected from elderly persons aged up to 100, and demonstrating no cognitive impairment, researchers isolated the immune cells whose antibodies are able to identify toxic beta-amyloid plaques, but not the amyloid precursor that is present throughout the human body and believed to play an important role in the growth of nerve cells. These immune cells were then cloned in large numbers and given intravenously to each patient just once a month. The good safety profile of the drug may well be attributed to its specific capacity to bond with the abnormally folded beta-amyloid protein fragment, as well as the fact that the antibody is of human origin. 165 patients with early-stage Alzheimer's were treated in the phase 1b clinical study. To further evaluate the safety and efficacy, two larger phase 3 clinical trials are now underway involving 2,700 patients from 20 countries in North America, Europe and Asia. The U.S. Food and Drug Administration has placed the drug on "Fast Track" designation, a program that supports the accelerated development of new treatments for serious conditions with an unmet medical need. "By collaborating with regulators through programs like Fast Track, we hope to bring effective treatments to patients and families affected by Alzheimer's disease as quickly as possible," said Dr. Sandrock. ---
Comments »
|