23rd July 2015 World's first bionic eye implant for a patient with macular degeneration U.S. firm Second Sight has announced that the first age-related macular degeneration patient has received its Argus II bionic eye at Manchester Royal Eye Hospital in the UK, as part of a ground-breaking study.
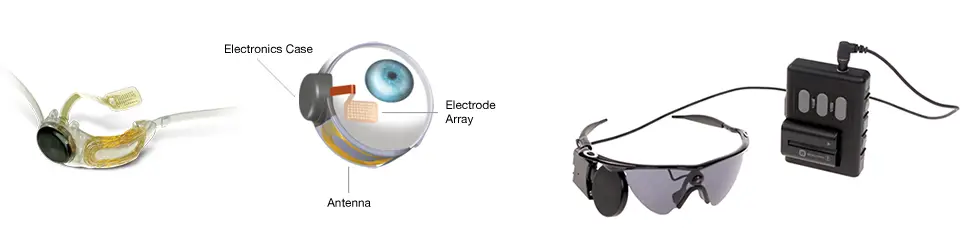
Second Sight – a developer of visual prosthetics – yesterday announced the first implant and successful activation of the Argus II Retinal Prosthesis System (Argus II) in a dry age-related macular degeneration (AMD) patient. Ray Flynn, 80, who has total loss of his central vision, can now make out the direction of white lines on a computer screen using the retinal implant. In an interview with the BBC, Mr Flynn said he was “delighted” with the implant and hoped in time it would improve his vision sufficiently to help him with day-to-day tasks like gardening and shopping. The implant is part of a feasibility study aiming to evaluate the safety and utility of the Argus II System in individuals with late-stage Dry AMD, a condition that severely affects central vision. The implant was performed at the Manchester Royal Eye Hospital in the United Kingdom by Dr. Paulo Stanga MD, Consultant Ophthalmologist & Vitreoretinal Surgeon. The device was activated approximately two weeks after implantation, and initial reports confirm that Flynn is receiving some useful vision. The Argus II has already been tested and approved in the United States and Europe for individuals with Retinitis Pigmentosa (RP) and Outer Retinal Degeneration, respectively. “The difference between RP and Dry AMD is that RP primarily affects the peripheral vision, whereas AMD primarily affects the central vision. Retinal implants for individuals with AMD may restore some useful vision in their central visual field, which is non-functional due to degeneration of the photoreceptors. The goal in restoring this central vision is to provide individuals with AMD more natural vision and ultimately improve their independence and quality of life," says Dr. Stanga. “This is totally ground-breaking research, where positive results from the study could provide advanced Dry AMD patients with a new alternative treatment.” The Argus II works by using a video camera mounted on sunglasses worn by the patient. This transmits images to a chip inside the eye, which shares the signals with an array of 60 electrodes (in a 6 × 10 grid). These electrodes convey electric fields to neural impulses, which are sent to the brain and interpreted as vision, restoring the ability to discern light, movement and shapes.
Eligibility for this study includes patients 25 to 85 years of age with advanced dry AMD, some residual light perception, and a previous history of useful form vision. Study subjects will be followed for three years to evaluate safety and utility of the Argus II system on visual function. Pending positive study results, the company plans to conduct a larger study to support market approvals. It is estimated that two million individuals worldwide are legally blind due to AMD and 375,000 people are blinded by RP. Second Sight Chief Executive Officer, Dr. Robert Greenberg, comments: “We are very excited to begin such an important study for this patient population and to have the opportunity to help a great deal more people living with blindness. Though it is obviously still early in this clinical trial, we are very encouraged by these initial results.” The launch of this study is another step toward Second Sight’s mission to enable blind people to achieve greater independence. Earlier this year, the first Orion I Visual Cortical Prostheses were implanted in animals to evaluate fit, form, stability, and biocompatibility. Human trials for the Orion I are planned to commence by Q1 2017. If successful, the Orion I has the potential to address nearly all forms of blindness.
Comments »
|