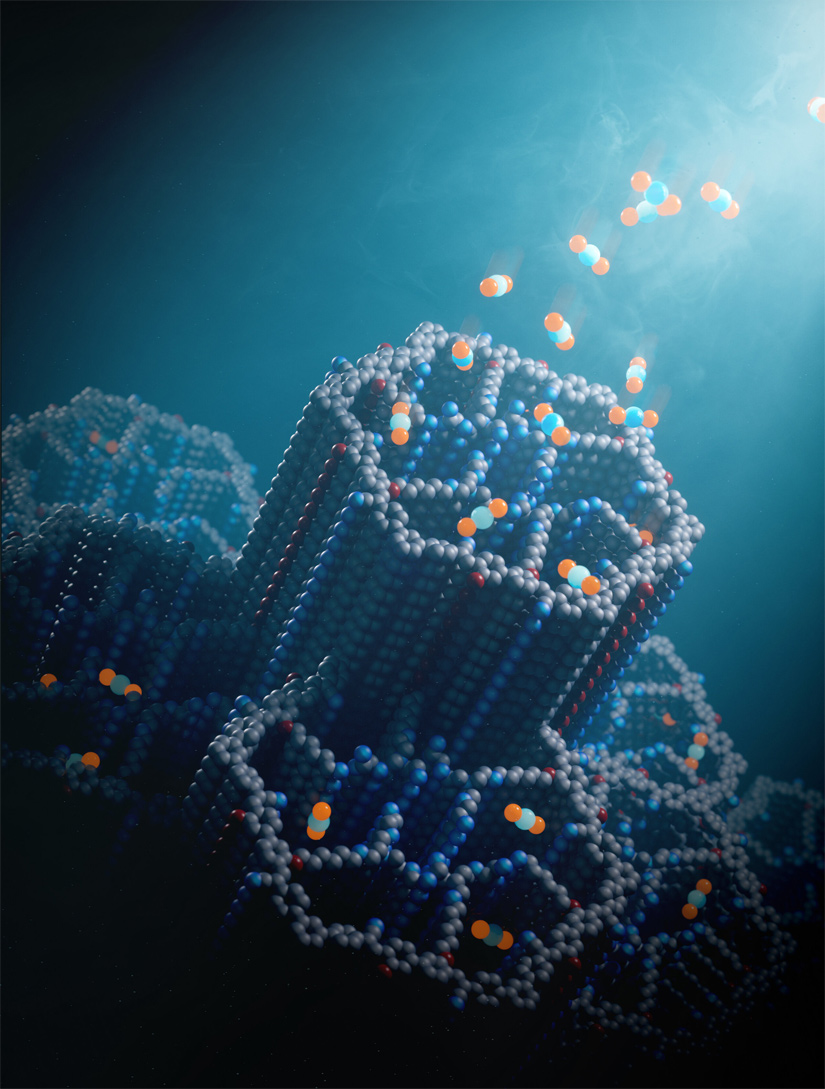
3rd November 2024 New material boosts CO2 capture efficiency A breakthrough material with a highly resilient, porous structure could accelerate efforts to achieve negative emissions.
Capturing and storing the carbon dioxide (CO2) produced from human activity is key to lowering atmospheric greenhouse gases and stabilising climate change. However, while today's carbon capture technologies work well for concentrated sources of carbon – such as power plant exhaust – they are less efficient at capturing CO2 from ambient air, where concentrations are hundreds of times lower than in flue gases. Yet direct air capture, or DAC, is being counted on to reverse the rise of CO2 levels, which have now reached 426 parts per million (ppm), about 50% higher than before the Industrial Revolution. Without it, according to the Intergovernmental Panel on Climate Change, limiting the global average temperature rise to 2 °C (3.6 °F) will be virtually impossible. In what seems to be a promising path forward, scientists at the University of California, Berkeley, have reported a new breakthrough in DAC. Their study, published in the prestigious journal Nature, describes a porous material known as a covalent organic framework (COF). This can absorb CO2 from ambient air without degradation by water or other contaminants, one of the major limitations of existing DAC technologies. "We took a powder of this material, put it in a tube, and we passed Berkeley air – just outdoor air – into the material to see how it would perform," explained Omar Yaghi, Professor of Chemistry at UC Berkeley. "And it was beautiful. It cleaned the air entirely of CO2. Everything. I am excited about it because there's nothing like it out there in terms of performance. It breaks new ground in our efforts to address the climate problem."
The new material could be substituted easily into carbon capture systems already deployed or being piloted, according to Yaghi. The material is so efficient that a mere 200 grams (0.4 lb) of it can sequester as much CO2 in a year – 20 kilograms (44 lb) – as an entire tree. "Flue gas capture is a way to slow down climate change because you are trying not to release CO2 to the air. Direct air capture is a method to take us back to like it was 100 or more years ago," said Zihui Zhou, a graduate student at UC Berkeley and study first author. "Currently, the CO2 concentration in the atmosphere is more than 420 ppm, but that will increase to maybe 500 or 550 before we fully develop and employ flue gas capture. So if we want to decrease the concentration and go back to maybe 400 or 300 ppm, we have to use direct air capture." The National Oceanic and Atmospheric Administration (NOAA), which monitors the CO2 level at dozens of stations around the world, recently updated its "pumphandle" animation with data up to 2024. While the increase in concentration since the Industrial Revolution appears rapid, it becomes a vertical line when viewed on a longer, geological timescale stretching back 800,000 years. Such abrupt changes in atmospheric CO2 are unprecedented in Earth's recent history, typically being associated with catastrophic events like massive volcanic eruptions or asteroid impacts. The last time CO2 levels hit 420 ppm, sea levels were several metres higher and ecosystems vastly different from those we know today.
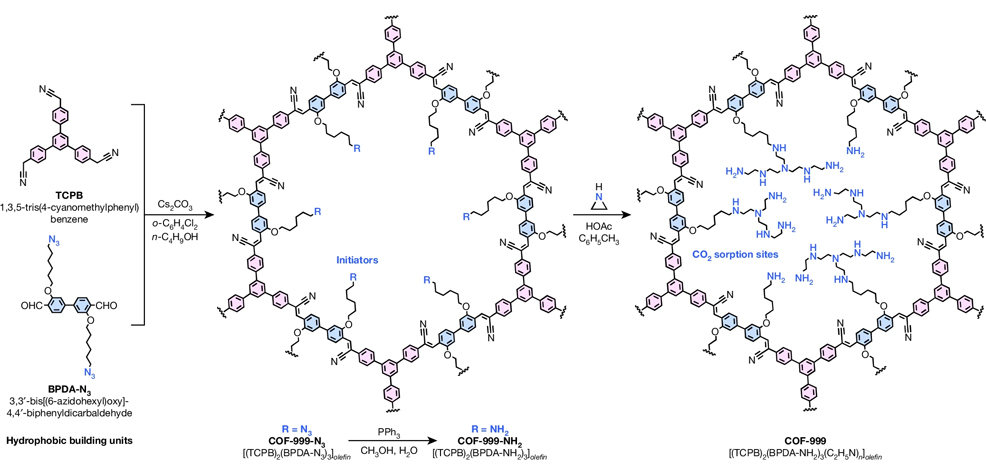
Professor Yaghi is the inventor of COFs and MOFs (metal-organic frameworks), both of which are rigid crystalline structures with regularly spaced internal pores that provide a large surface area for gases to stick or adsorb. Some MOFs that his lab have developed can adsorb water from the air, even in arid conditions, and when heated, release the water for drinking. He has been working on MOFs to capture CO2 since the 1990s, long before most people had even heard of DAC. Two years ago, Yaghi's team created MOF-808 – a very promising material that adsorbs CO2. But the researchers found that after hundreds of cycles of adsorption and desorption, the MOFs broke down. Working with colleagues including Zhou, Yaghi discovered why some MOFs degrade for DAC applications, and began to design a stronger material, which they call COF-999. As with MOF-808, the pores of COF-999 are decorated inside with amines, which are NH2 groups (a nitrogen atom bonded to two hydrogen atoms), allowing the uptake of more CO2 molecules. But whereas MOFs are held together by metal atoms, COFs are held together by covalent carbon-carbon and carbon-nitrogen double bonds, among the strongest chemical bonds in nature. "Trapping CO2 from air is a very challenging problem," explains Yaghi. "It's energetically demanding, you need a material that has high carbon dioxide capacity, that's highly selective, that's water stable, oxidatively stable, recyclable. It needs to have a low regeneration temperature and needs to be scalable. This COF has a strong chemically and thermally stable backbone. It requires less energy – and we have shown it can withstand 100 cycles with no loss of capacity. No other material has been shown to perform like that. It's basically the best material out there for direct air capture." Yaghi is optimistic that artificial intelligence can help speed up the design of even better COFs and MOFs for carbon capture or other purposes, specifically by identifying the chemical conditions required to synthesise their crystalline structures: "We're very, very excited about blending AI with the chemistry that we've been doing," he said.
Comments »
If you enjoyed this article, please consider sharing it:
|
||||||