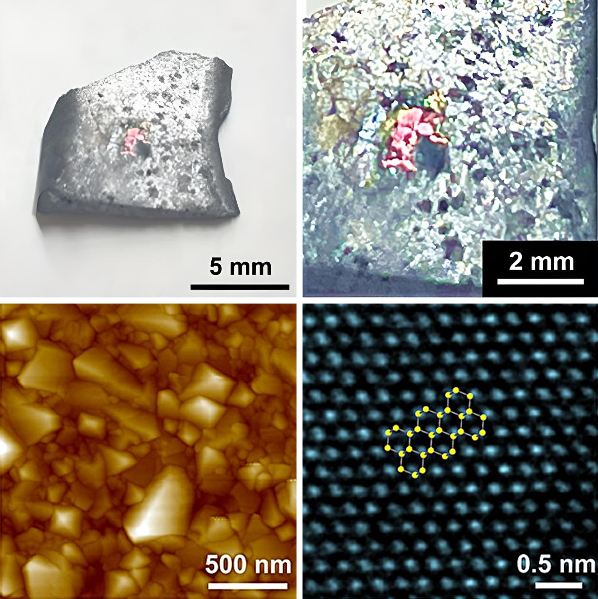
27th April 2024 Synthetic diamonds created under ambient pressure A team of scientists from South Korea has demonstrated the creation of synthetic diamond in just 150 minutes, without needing seed particles and using only 1 atmosphere of pressure.
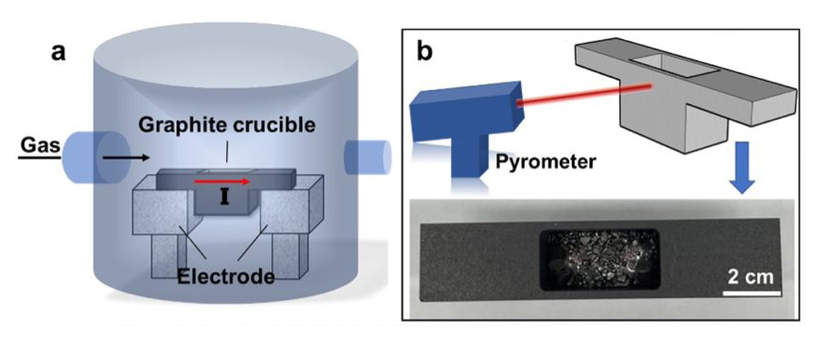
It takes between 1 and 3.3 billion years for diamonds to form naturally, which is 22% to 73% of Earth's age. The process occurs deep within Earth's mantle, approximately 150 to 250 kilometres below the surface, where temperatures can reach 1,400 °C (2,550 °F) and pressures can exceed 6 gigapascals (GPa), equivalent to 60,000 times atmospheric pressure. As a further comparison, even the bottom of the Mariana Trench, the deepest point of any ocean, has a pressure of "only" 0.1 GPa. Under these extreme conditions, carbon atoms bond together in a specific crystalline structure. The resulting material is eventually carried upwards through geologic activity such as volcanoes. In addition to its popularity as a gemstone, diamond is an essential component for industrial applications like cutting and polishing tools, due to its hardness and thermal conductivity being the highest of any natural material. Synthetic diamonds, invented in 1954, are made in the laboratory much faster than natural diamonds, usually within a few days to several weeks, depending on the desired quality. However, these still require high-pressure and high-temperature methods, while being limited to only a few carats. Now, a team from the Institute for Basic Science, a Korean government-funded research institute, has grown diamonds at 1 standard atmosphere (atm) and a significantly lower temperature of 1,025 °C (1,877 °F) in just 150 minutes. Their study is reported this week in the peer-reviewed journal Nature. The scientists developed a cold-wall vacuum system, which allows for rapid heating and cooling. They carefully mixed and placed a liquid metal alloy composed of gallium, iron, nickel, and silicon onto a graphite crucible inside the device. Two water-cooled electrodes had a current applied to them, heating the crucible at a rate of 7.7 °C (13.9 °F) per second. Meanwhile, a gas pipe delivered methane and hydrogen into the chamber.
Under these conditions, carbon atoms from the methane diffused into the liquid metal. Remarkably, after just 15 minutes, small fragments of diamond crystals began to form just beneath the surface, while 150 minutes of exposure produced a continuous diamond film. The team had previously developed a larger device, with a volume of 100 litres. However, bigger is not always better. This earlier version took much longer to reset between experiments, requiring three hours of configuration. Rod Ruoff, a world-renowned expert on nanotechnology, directed the project. He asked colleagues to design and build a much smaller chamber to greatly reduce the setup time: "Our new homebuilt system (named RSR-S), with an interior volume of only 9 litres, can be pumped out, purged, pumped out, and filled with methane/hydrogen mixture, in a total time of 15 minutes," Mr. Ruoff explains in a news release from the institute. "Parametric studies were greatly accelerated, and this helped us discover the parameters for which diamond grows in the liquid metal." "One day with the RSR-S system when I ran the experiment and then cooled down the graphite crucible to solidify the liquid metal, and removed the solidified liquid metal piece, I noticed a 'rainbow pattern' spread over a few millimetres on the bottom surface of this piece," said Yan Gong, a graduate student from the Ulsan National Institute of Science and Technology, and first author of the study. "We found out that the rainbow colours were due to diamonds! This allowed us to identify parameters that favoured the reproducible growth of diamond." The initial formation occurs without the need for diamond or other seed particles commonly used in conventional high-pressure and high-temperature (HPHT) or chemical vapour deposition methods. Once formed, the diamond crystals merge to form a film, which can be easily detached and transferred to other substrates, for further studies and potential applications. Synchrotron two-dimensional X-ray diffraction measurements confirmed that the film has a very high purity of the diamond phase. The team also discovered that silicon plays a critical role in this new growth of diamond. The size of the grown diamonds became smaller and their density higher as the concentration of silicon in the alloy increased from the optimal value. Diamonds could not be grown at all without the addition of silicon, suggesting that silicon may be involved in the initial nucleation of diamond. At present, this method is limited to thicknesses of just a few hundred nanometres. The researchers note that it may find applications in magnetic sensing and quantum computing. However, they suggest that with straightforward modifications, it could be improved and scaled up, expanding the potential range of uses. "Our discovery of nucleation and growth of diamond in this liquid metal is fascinating and offers many exciting opportunities for more basic science," adds Ruoff.
Comments »
If you enjoyed this article, please consider sharing it:
|
||||||