15th February 2024 New cells may cut lab-grown meat costs A breakthrough in cultured meat has been reported at Tufts University, Massachusetts. Bovine muscle cells can now be made to produce their own growth signals, removing the costly ingredients from the production process.
Cellular agriculture – the production of meat from cells grown in bioreactors rather than harvested from farm animals – has already seen major strides in technology making it a more viable option for the food industry. Yet another such leap has now been made at the Tufts University Center for Cellular Agriculture (TUCCA). Led by David Kaplan, Professor of Engineering, a team of researchers have created bovine (beef) muscle cells that produce their own growth factors, which could potentially slash the costs of production. Growth factors, whether used in laboratory experiments or for cultivated meat, bind to receptors on the cell surface and provide a signal for cells to grow and differentiate into mature cells of different types. In their study, published in the journal Cell Reports Sustainability, researchers modified stem cells to produce their own fibroblast growth factor (FGF) which triggers growth of skeletal muscle cells, the kind found in a steak or hamburger. "FGF is not exactly a nutrient," said the project's lead researcher Andrew Stout, who holds a PhD in Biomedical Engineering/Cellular Agriculture. "It's more like an instruction for the cells to behave in a certain way. What we did was engineer bovine muscle stem cells to produce these growth factors and turn on the signalling pathways themselves."
90% of the production costs
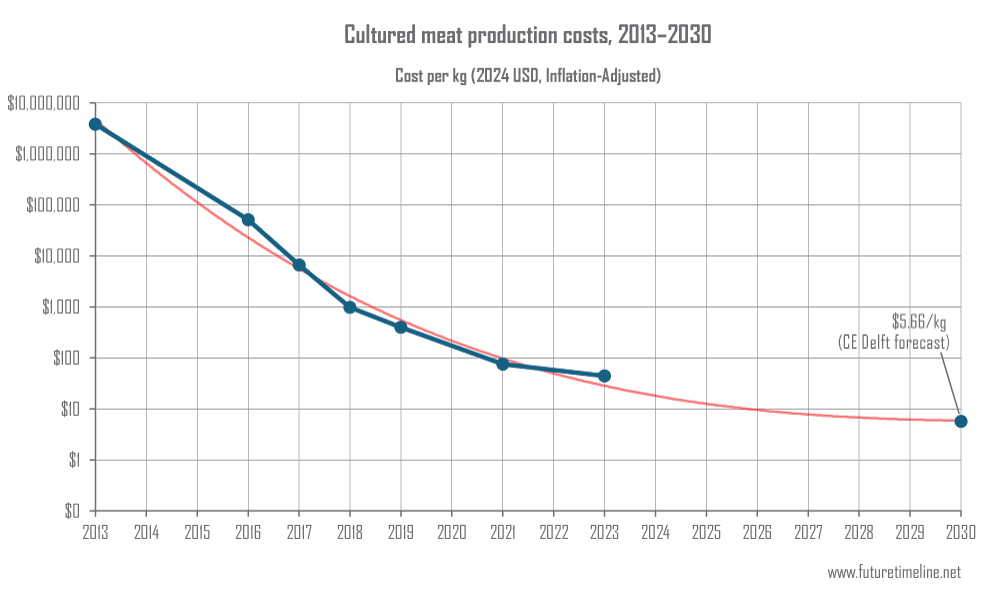
Until now, growth factors had to be added to the surrounding liquid, or media. Made from recombinant protein and sold by industrial suppliers, they can in some cases account for over 90% of the production costs in cellular agriculture. Since the growth factors don't last long in the cell culture media, they also have to be replenished every few days. This limits the ability to provide an affordable product to consumers. Taking that ingredient out of the growth media could lead to potentially enormous cost savings. Scientists unveiled the world's first lab-grown beef burger at an event in London back in 2013. This had a production cost of $330,000 (or 8.8 times that figure when measured on a per kilogram basis, as on the graph below). Since then, cultured meat has followed a trend remarkably similar to Moore's Law, solar power, and other exponential technologies like genome sequencing costs. Today's bioreactor facilities are now starting to achieve below $100 per kilogram and their production costs could reach as low as $5.66/kg by 2030, according to a forecast by Dutch consultancy CE Delft. In an interview with Reuters last year, the president of U.S. multinational food processing corporation Archer-Daniels-Midland stated that for cultured meat to be price competitive, the production cost (as opposed to wholesale or retail price) must reach $6.42/kg ($2.92 per lb). The new technique developed at TUCCA may help to accelerate its timeline from R&D to commercialisation and enable a more rapid scaling up.
"While we significantly cut the cost of media, there is still some optimisation that needs to be done to make it industry-ready. We did see slower growth with the engineered cells, but I think we can overcome that," said Stout. Future strategies may include changing the level and timing of expression of FGF in the cell or altering other cell growth pathways. "In this strategy, we're not adding foreign genes to the cell, just editing and expressing genes that are already there, to see if they can improve growth of the muscle cells for meat production," he added. That approach may also lead to simpler regulatory approval of the ultimate food product, since regulation is more stringent for the addition of foreign genes vs editing of native genes. Could the strategy work for other types of meat, such as chicken, pork, or fish? Stout believes so: "All muscle cells and many other cell types typically rely on FGF to grow." He envisions the approach being applied to other meats, although there may be variability for the best growth factors in different species. "Work is continuing at TUCCA and elsewhere to improve cultivated meat technology," said Kaplan, "This includes exploring ways to reduce the cost of nutrients in the growth media, and improving the texture, taste, and nutritional content of the meat. Products have already been awarded regulatory approval for consumption in the U.S. and globally, although costs and availability remain limiting. I think advances like this will bring us much closer to seeing affordable cultivated meat in our local supermarkets within the next few years." Cultured meat has the potential to be massively disruptive in the food industry – offering major benefits for the environment and human health, as well as preventing animal cruelty and slaughter. It could dramatically reduce land and water use, greenhouse gas emissions and other pollution. Cleaner protein sources would become possible, free from antibiotics and potentially harmful bacteria. Its mainstream adoption could mark a pivotal step towards a more sustainable and humane food system.
Comments »
If you enjoyed this article, please consider sharing it:
|
||||||