23rd September 2019 Alzheimer's symptoms reversed by head-worn device using electromagnetic waves A small clinical trial, announced by U.S. company NeuroEM Therapeutics, shows reversal of cognitive impairment in Alzheimer’s disease patients after just two months of treatment using a wearable head device. Electromagnetic waves emitted by the device appear to penetrate the brain to break up amyloid-beta and tau deposits.
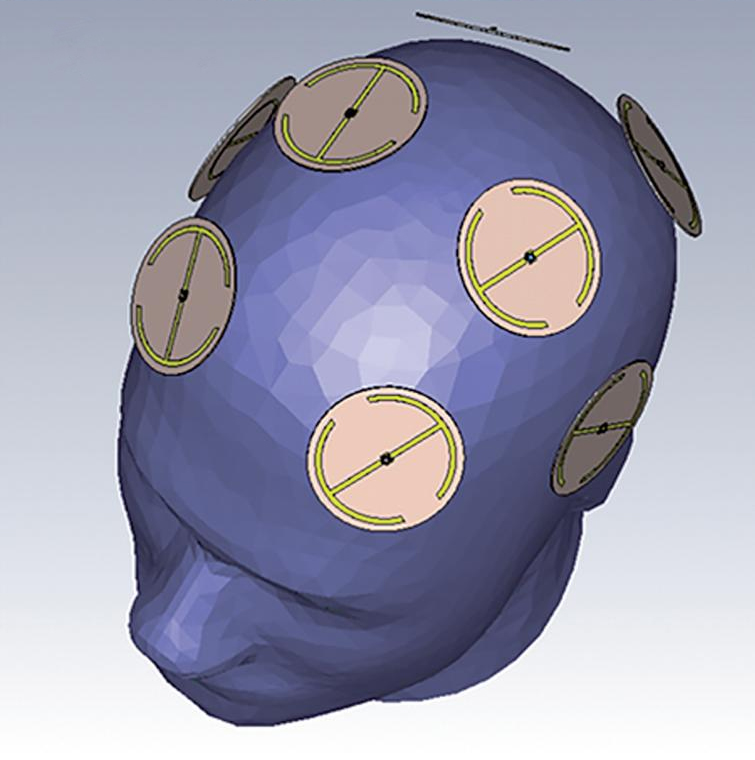
There is finally some encouraging news for the millions of people suffering from Alzheimer's disease (AD). NeuroEM Therapeutics, headquartered in Phoenix, Arizona, has announced findings from an open-label clinical trial showing reversal of cognitive impairment in Alzheimer's disease patients after just two months of treatment using the company's wearable head device for in-home treatment. The results demonstrate that Transcranial Electromagnetic Treatment (TEMT) was safe in all eight participating patients with mild to moderate AD and led to enhanced cognitive performance in seven of them, as measured by their ADAS-cog score, a benchmark for testing AD therapeutics. The study is published in the Journal of Alzheimer's Disease. The researchers had previously demonstrated that treating mice with electromagnetic waves in the radio frequency range offered protection against memory impairment in young mice with AD, along with reversal of memory impairment in aged AD mice. For the present clinical study in humans, they used the same treatment (twice daily for 1 hour) via the MemorEM head device pictured above. This has multiple, highly-specialised emitters positioned within a head cap that are activated sequentially, with treatments easily administered in-home by the patient's caregiver. Additionally, the device allows for near-complete mobility to perform the vast majority of household activities during treatments.
"Perhaps the best indication that the two months of treatment was having a clinically-important effect on the Alzheimer's patients in this study is that none of the patients wanted to return their head device after the study was completed", said Dr. Gary Arendash, CEO of NeuroEM Therapeutics. "One patient even exclaimed 'I've come back.'" All were offered and accepted continued TEMT in a now on-going extension study. In their journal paper, the investigators have published strong evidence that TEMT is directly affecting the Alzheimer's disease process by easily penetrating the brain and its cells to break up aggregates of two toxic proteins, called amyloid-beta and tau. The electromagnetic waves' ability to disaggregate both of these proteins inside neurons appears to be key to stopping and reversing cognitive loss. Present drugs in clinical trials have great difficulty getting into the brain and then into brain cells. Even if they succeed in doing so, they do not yet have the capacity to precisely target the small aggregates of A-beta and tau proteins.
"Despite significant efforts for nearly 20 years, stopping or reversing memory impairment in people with Alzheimer's disease has eluded researchers," said Amanda Smith, Director of Clinical Research at the University of South Florida Health, Byrd Alzheimer's Institute, the clinical centre for the study. "These results provide preliminary evidence that TEMT administration we assessed in this small AD study may have the capacity to enhance cognitive performance in patients with mild to moderate disease." After two months of treatment administered, none of the eight patients exhibited any harmful side effects. Furthermore, the post-treatment brain scans revealed no visible induction of tumours or brain bleedings ("microhaemorrhages"). Using the ADAS-cog benchmark to test a variety of cognitive measures, seven of the eight patients had a 4+ point increase in performance, indicating a very large and clinically-important effect. Since AD patients typically show a 4+ point decline in ADAS-cog performance over a given one year period, the 4+ point improvement was as if the treated patients had gone back in time one year earlier. MRI brain scans of individual AD patients also revealed evidence of increased communication between neurons in a brain area critical for cognitive integration called the cingulate cortex/cingulum. The researchers believe that TEMT may be "an entirely new therapeutic intervention against Alzheimer's" and that "Neuro EM's bioengineering technology may be succeeding where drug therapy against this devastating disease has thus far failed." NeuroEM Therapeutics is now planning the next phase of clinical trials, with a larger cohort of 150 subjects being recruited later this year for treatment with the company's MemorEM device. If that trial shows continued safety and cognitive benefits, the company will apply to the FDA for approval of the device for treatment of AD.
Comments »
If you enjoyed this article, please consider sharing it:
|