7th January 2021 Gene-editing method shows promise for premature aging syndrome Scientists have fixed a genetic mutation in mice with progeria, a rapid aging disease. The treatment could one day be used in humans who would otherwise die in childhood. Approximately 1 in 4 million children are diagnosed with progeria within the first two years of birth, and virtually all of these children develop health issues in childhood and adolescence that are normally associated with old age – including cardiovascular disease (heart attacks and strokes), hair loss, skeletal problems, subcutaneous fat loss and hardened skin. For this new study, published yesterday in Nature, researchers used a breakthrough DNA-editing technique called base editing. This substitutes a single DNA letter for another without damaging the DNA. Progeria is caused by a rare mutation (discovered in 2003) in the nuclear lamin A gene, in which one DNA base C is changed to a T. This change increases the production of a toxic protein called progerin, which causes the rapid aging process. Researchers used the base editing method to study how changing this mutation might affect progeria-like symptoms in mice.
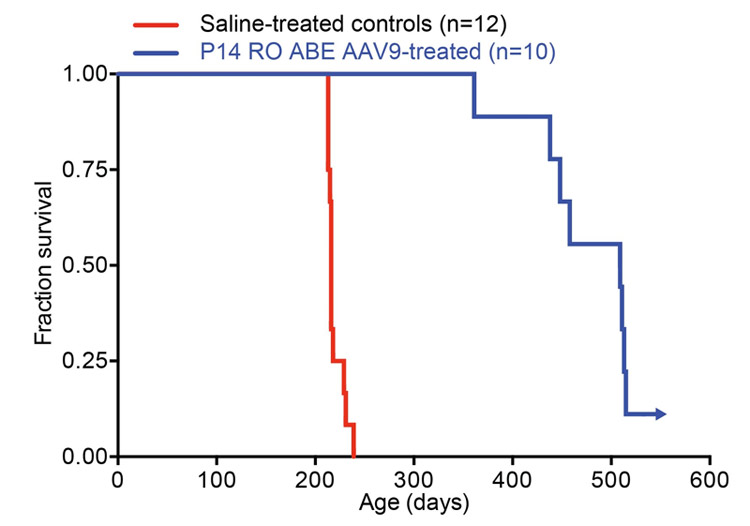
"The toll of this devastating illness on affected children and their families cannot be overstated," said Francis Collins, Ph.D., a senior investigator at the U.S. National Human Genome Research Institute (NHGRI) and a corresponding author on the paper. "The fact that a single specific mutation causes the disease in nearly all affected children made us realise that we might have tools to fix the root cause. These tools could only be developed thanks to long-term investments in basic genomics research." Co-author David Liu, Ph.D., and his lab at the Broad Institute developed the base-editing method in 2016, funded in part by NHGRI. Unlike CRISPR, which makes double-strand cuts in DNA, the base editor used in the progeria study nicks just one strand and swaps out a single base. "CRISPR editing, while revolutionary, cannot yet make precise DNA changes in many kinds of cells," said Dr. Liu. "The base-editing technique we've developed is like a find-and-replace function in a word processor. It is extremely efficient in converting one base pair to another, which we believed would be powerful in treating a disease like progeria." To test the effectiveness of their method, the team initially obtained connective tissue cells from progeria patients. They used their base editor on the LMNA gene within the patients' cells in a laboratory setting. The treatment was highly efficient and fixed the mutation in 90% of cells. Following this success, they moved onto animal studies. The researchers delivered a single intravenous injection of the DNA-editing mix into nearly a dozen mice with the progeria-causing mutation soon after birth. The gene editor successfully restored the normal DNA sequence of the LMNA gene in up to 60% of cells in various organs, including the heart and aorta. Many of the mice cell types still maintained the corrected DNA sequence for as long as six months after treatment. In the aorta, the results were even better than expected as the edited cells seemed to have replaced those that carried the progeria mutation and dropped out from early deterioration. Most dramatically of all, the treated mice's lifespan increased from seven months to almost 1.5 years. The average lifespan for normal mice is two years.
"As a physician-scientist, it's incredibly exciting to think that an idea you've been working on in the laboratory might actually have therapeutic benefit," said Jonathan Brown, M.D., study co-author and assistant professor of cardiovascular medicine at Vanderbilt University Medical Center. "Ultimately our goal will be to try to develop this for humans, but there are additional key questions that we need to first address in these model systems." "These findings show that a one-time base editor injection can directly correct the mutation and rescue of several of the consequences of the disease," added Liu. "Additional work optimising the editor, delivery vector, dosing, and timing towards potential clinical use are underway." This study follows another recent milestone, as the Food and Drug Administration (FDA) approved the first treatment for progeria in November 2020 – a drug called lonafarnib. The drug therapy provides some life extension, but is not a cure. The new DNA-editing method reported here may provide an additional and more dramatic treatment option in the future.
Comments »
If you enjoyed this article, please consider sharing it:
|